Monday, June 21, 2010
Monday, June 14, 2010
Majestic Saturn
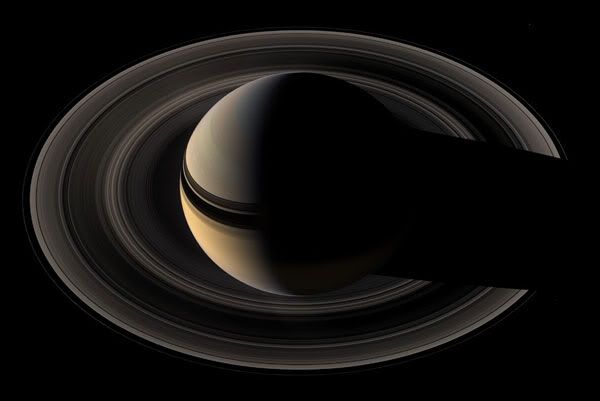
( Click for more! )
More full sized images and some more details are on Boston.com's The Big Picture.
Top 10 most interesting multiple star systems
(In no particular order.)

 Albireo
AlbireoA firm favourite among many amateur astronomers, Albireo (also known as beta-cygni) is actually a triple star system. Through a telescope though, it appears as a particularly beautiful visual binary -- a bright yellowish star with a fainter blue companion (shown here to the right). In 1976, astronomers at Kitt Peak discovered that the bright yellow star is actually a spectroscopic binary.
The two visual stars are around 380 light years away, and a huge 35 arcseconds apart. In other words, that fainter blue star (a fast rotating Be-class star) would take over 100,000 years to complete one orbit of the central binary -- assuming it's even part of the system.
Cygnus X-1
Cygnus X-1 is a very interesting little system. Discovered in 1964, it's one of the brightest x-ray sources in the sky, with a flux as high as 2.3x10-23 janskys (if you don't know what a jansky is, suffice to say that's surprisingly bright).
In fact, Cyg X-1 is one of the most promising candidates in the sky for a stellar mass black hole, with around 8.7 solar masses compressed into an estimated 26km event horizon. In a tight binary orbit (around 0.2AU) around the black hole, a blue supergiant orbits once every five and a half days in a nearly perfect circular orbit. It is slowly being ingested, as stellar material is drawn towards the black hole, forming an accretion disk. As the inner parts of this disk are heated to millions of kelvins, the atoms start to emit the bright x-rays as seen from Earth. Around 6000 light years away, there's a fair chance it could be part of the Cygnus OB3 association, which would make the system around 5 million years old. The black hole may have once been a star over 40 solar masses in size.
The evidence for Cyg X-1 being a black hole is actually so good that Stephen Hawking once lost a bet against Kip Thorne, acquiescing in 1990 that it could only be a gravitational singularity!
16 Cygni
70 light years away, 16 Cygni is a trinary system. A Sun-like yellow dwarf with a close(ish) red dwarf companion orbiting 73 AU away. Further out, another, slightly smaller, yellow dwarf orbits the main pair. Further out, but no one's entirely sure how far exactly, it would seem. Estimates range from 860 AU to 15,180 AU, giving an orbital period of anywhere between 18,200 to 1.3 million years. Another good example of how a tiny change in the calculations and observations can have a dramatic effect in astronomy. The 16 Cygni system is estimated at around 10 billion years old (so quite a bit older than The Sun), and has one known planet. Orbiting the smaller of the two yellow stars is a massive planet in a close elliptical orbit.
Interestingly, 16 Cygni is one of a select few stars which has had messages beamed to it via METI. If there's anyone there to hear it, they should receive it in November 2069. Who knows... With all the messages being send out, we might even have a reply sometime over the next 200 years or so.
Kelu-1
Quite nearby at only 30 light years away, Kelu-1 was only discovered recently. The reason why is simply -- it's a vanishingly faint brown dwarf system. Discovered in 1997, Kelu-1 was one of the first known free brown dwarfs not to be tied to a larger stellar companion. Since then, it's been visually confirmed that there are actually two visible brown dwarfs in the system.
In 2008 though, it was found that actually, it's probably a trinary system, with the "larger" of the two stars actually being a spectroscopic binary. The central pair, Kelu-1Aa and Kelu-1Ab are difficult to discern, but the orbiting dwarf Kelu-1B is around 6.5 AU from them, completing an orbit once every 38 years or so.
 Pismis 24-1
Pismis 24-1In a stark contrast to the tiny Kelu-1 system, Pismis 24-1 is one of the heaviest trinary systems known. Interestingly, it's in very much the same configuration, with a spectroscopic binary pair too close to properly resolve, and another star at a somewhat greater distance.
It's part of the Pismis 24 open cluster -- a collection of some of the most massive stars known Pismis 24-1 is the brightest star visible in the picture to the left. Before it was discovered that this star is actually a multiple star system, it was estimated at nearly 300 solar masses. A single star simply cannot form with such a high mass -- it would be above the Eddington Limit. As you can imagine, this baffled a few astrophysicists for a while. All the same, three stars each weighing in at around 100 solar masses is still rather immense, but at least it's explainable by theories!
Castor
Located in the constellation of Gemini, Castor is a hexuple star system. In other words, it contains a whopping 6 stars! Visually, you can discern two separate stars, but each of them is also a spectroscopic binary. Both of these are actually an A-class star with a red dwarf companion. Another, fainter, companion star to the system is quite a rarity, in that it's an eclipsing binary system composed of two red dwarfs.
Quite a big family, as stars go.
40 Eridani
Just over 16 light years away, the 40 Eridani system is the home of the first white dwarf ever discovered. It's an interesting system because that white dwarf would once have been the main star observable. As a white dwarf, it's now little more than a stellar corpse, left orbiting the stars that were once its underlings. It now revolves in an eccentric orbit around 35AU from a red dwarf flare star companion. These two both orbit 40 Eri A, a red-orange dwarf star, roughly 400AU away.
There's a fair chance a habitable planet could exist in the 40 Eridani system. The habitable zone around the main star is around 0.6AU away (more or less the same orbit as Venus around the Sun). The other two stars would have little influence on such a planet, appearing as a bright red and white pair -- not bright enough to illuminate the planet at night, but bright enough to be visible during the day (assuming an Earth-like atmosphere). They'd appear as roughly magnitude -8 and -6 white and reddish stars in the sky. To compare, Venus is around magnitude -4.7 at its brightest.
Polaris
Polaris is surely amongst the most famous stars in the sky. 430 light years away, it's been used by sailors in the Northern hemisphere for hundreds (perhaps thousands) of years to navigate by. In fact, Polaris is actually another trinary system. The primary star in the system is a yellow bright giant. It's orbited closely by a hard-to-see dwarf star at around 18.5 AU, and more distantly by an F-class main sequence star, a huge 2400 AU away. The optimist in me would like to point out that an F-class star with solar metallicity could well be host to a habitable planet or two, though it's hard to say how safe it is being so close to a variable giant!
Polaris is another star which is farily popular among amateur observers. It's a classic population I cepheid variable (actually the closest one to Earth), and though I've never looked at it myself, I've heard others say that with a good enough telescope, you can even make it out as a visual binary.
TV Crateris
150 light years distant, TV Crateris (also known as HD 98800) is an interestting little quadruple star system. Firstly, all four stars are T Tauri stars (young stars, still not properly formed) and secondly, they all appear to be sun-like stars. Two close binary pairs orbit each other at around 50 AU, and as you'd expect from a young star system, there's a big dusty accretion disk surrounding the central pair.
Excitingly though, that disk isn't constant. It has a big clearing. Specifically, it's made up of an inner disk from 1.5 - 2 AU and an outer disk that starts around 5.9 AU. A big gap in a ring like that could quite possibly be caused by planets, either fully formed or still accreting material. Which is a logical assumption -- dusty disks are often associated with planets. It could well be that we're seeing planet formation happening in this system. If we are, they're going to be brightly lit planets for any life that might someday live there...
Alpha Centauri
Including Alpha Cen is almost obligatory in this list! A trinary system, I've written about it before at great length, as have many others. With good reason. Frankly, our nearest stellar neighbour, at a mere 4.37 light years away, is quite an inspiring place -- if for no other reason than that it's almost certainly going to be the first extrasolar system the human race eventually visits.
Appearing as a single star to the unaided eye, Alpha Centauri is the third brightest star in the sky. With a telescope though, it makes for a very pretty visual binary. Dim little Proxima, the third star in the system, appears about 2.2° away from the central pair. If it were bright enough to be seen by the naked eye, you wouldn't even think it was part of the same system.
Standing on a rocky moon somewhere in the Alpha Centauri system might grant you a view a bit like this one...

Image credits (in order of appearance):
"Tatooine planet" - NASA JPL/Caltech
Albireo - Richard Yandrick
Pismis 24 open cluster - NASA, ESA and J. M. Apellániz
Alpha Centauri hypothetical planet - "The plague", Wikimedia Commons
8 reasons to be amazed by Gamma Ray Bursts!

1. There are "long bursts"...
Long bursts are widely believed to be a type of supernova event. In fact, some have been observed in association with a supernova (the first being GRB 980425). The process is suitably melodramatic. As soon as an extremely massive, fast rotating star burns enough fuel, it's core becomes so massive that it can't withstand it's own gravity. It collapses, in a similar way to a regular supernova, but it doesn't stop there. Because these stars are so massive, more and more stellar material is sucked into the core, collapsing it directly into a black hole. The star then proceeds to devour itself, releasing such vast amounts of energy that two beams of energy puncture the star at it's North and South poles. Think about that for a moment. Enough energy to puncture a star. A massive one at that!It's one of these beams of intensely bright radiation that we see as a "Gamma Ray Burst".
2. ...and there are "short bursts"
Short gamma ray bursts are believed to come from something altogether different. While there are competing theories and diagreement, many believe that short bursts come from two neutron stars colliding. While neutron stars are so tiny, you might not expect them to ever come close enough to collide, don't forget that an average neutron star will be twice as massive as the Sun and have gravity to match.If two neutron stars get trapped together by gravity, it's thought that they would slowly and inexorably fall together. As they rotate around each other, they would emit gravitational waves, losing energy and thus losing speed. With the loss of speed, they'd lose orbital distance. Eventually (probably over millions of years), they'd fall so close together that they'd crash into each other. The collision would be violent, but swift, emitting a brief but gargantuan belch of gamma rays before both stars collapsed into a silent black hole.
3. (Actually, there are other types too)
There's a third theory which is sometimes accepted as the cause of some gamma ray bursts. Megaflares.One day before my 24th birthday in 2004, a blast of gamma radiation saturated every single gamma ray satellite in orbit. It was so powerful it even disrupted Earth's ionosphere. The source? A type of rare, highly magnetised neutron star, known as a magnetar. The specific one, SGR 1806-20, is one of only five known in our entire galaxy.
Whether megaflares could be the source of some GRBs or not is disputed, but one thing's for certain. A megaflare like the one in 2004 would be easily observable in another galaxy.
4. You can see them from halfway across the Universe
 Gamma ray bursts don't just emit gamma rays. They also have an "afterglow" that covers the entire electromagnetic spectrum. X-rays and ultraviolet right the way down to infrared and radio waves.
Gamma ray bursts don't just emit gamma rays. They also have an "afterglow" that covers the entire electromagnetic spectrum. X-rays and ultraviolet right the way down to infrared and radio waves.In March 2008, one burst (GRB 080319B) was so bright that you would have been able to see it with the naked eye for 30 seconds. Despite a redshift of 0.937. Let's put that into perspective -- because of the way redshifts work, a redshift of 1 can be considered to be about halfway across the observable Universe. Loosely 7.5 billion light years away. This GRB was half a Universe away and visible to the naked eye! Incredible.
5. We've found one from shortly after the Universe was born!
The record holder for most distant object ever observed was a gamma ray burst. Just last month, GRB 090423 was picked up by the Swift satellite. It lasted about 10 seconds, and was measured to have a redshift of 8.2. Whatever caused it exploded roughly 13 billion years ago -- estimated at a mere 630 million years after the Big Bang. The Universe was just a baby back then. Even the galaxies were young that long ago.Interestingly, seeing a GRB this old implies that massive stars were indeed forming and dying in the early Universe. It was probably stars like these that created the heavier elements that would eventually form cosmic dust, planets... and us.
6. We've known about them since the Cold War
We've actually known about gamma ray bursts since 1967. In 1963, the Partial Test Ban Treaty was drawn up to prevent the deployment of nuclear weaponry in outer space and the upper atmosphere (as well as under water). In order to monitor this, the US military developed Project Vela.Instead of detecting nuclear weapons though, project Vela detected GRB 670702! After several more were observed, the Los Alamos National Laboratory published the paper Observations of Gamma-Ray Bursts of Cosmic Origin -- the first ever publication on GRBs. Since then, GRB research has blossomed into a research field in it's own right.
7. They're rather dangerous
Ok, that's an understatement. On the list of "Things which could cause the end of the world", GRBs are pretty high up there.When we talk about GRBs, we're talking about something violent enough that we can see them from the other side of the Universe. Think about it. That isn't something you'd want to happen in your backyard. It's a worthwhile concern though. A nearby gamma ray burst would severely damage Earth's biosphere. Those gamma rays, much tougher than the worst solar flare imaginable, would fry the upper atmosphere. Nitrogen and oxygen would be forcibly reacted into nitric oxide, and nitric oxide is sadly rather good at destroying ozone. It's been calculated that a nearby GRB hitting Earth for just 10 seconds could destroy up to half of Earth's ozone. It would take at least five years for the ozone layer to recover, during which time ultraviolet from the Sun would play havoc with life on land.
Worryingly, it's even been considered that the Ordovician-Silurian extinction events 450 million years ago could have been caused by just such a gamma ray burst.
8. We're safe. For now. So we think.
 GRBs are mercifully rare. Some estimate that just one burst will occur in the Milky Way every hundred thousand years to every million years. Others believe that bursts can only happen in galaxies with much fewer heavy elements than the Milky Way ("Metal-poor" galaxies).
GRBs are mercifully rare. Some estimate that just one burst will occur in the Milky Way every hundred thousand years to every million years. Others believe that bursts can only happen in galaxies with much fewer heavy elements than the Milky Way ("Metal-poor" galaxies).Some astronomers were concerned for a while about a star named WR 104 (shown in this image here), an ageing massive star (known as a Wolf-Rayet star). The trouble is, we don't know enough about gamma ray bursts to say if this star could produce one. If it could, the star's rotational axis is aligned within about 16° of Earth which would make this a rather dangerous place to be living. Reassuringly, most now believe that WR 104 it isn't the "loaded gun" we once thought it was, and is unlikely to produce a gamma ray burst.
But there's no way we can really know for certain...
Didn't we have a lovely time, the day we went to Mare Tranquillitatis...
But if you don't do it,
you come to a standstill
and progress is halted."
I must say, I agree with Greg Fish's recent post over at World of Weird Things. In fact, I agree rather vehemently. I've bemoaned in the past, how human technology seems to be stagnating in many areas. Indeed, in that same rant almost a year ago, I drew reference to how the proposed Orion Spacecraft, part of NASA's Constellation program looked suspiciously similar to the, frankly, old fashioned space capsules that were used back during the early days.
Now granted, NASA have upgraded the design a bit since then, using newer technology. They're going to make them a little more spacious too, holding four to six astronauts. A definite improvement over the first space capsules. I've heard it said that the old Gemini spacecraft were so cramped that they weren't so much piloted as worn. But all the same, the fact remains that it's a capsule. A pressurised tin can. Quite a far cry from the feat of engineering that should be replacing the Space Shuttle's magnificent design. Instead, we're using new technology in an old design. A bit like rebuilding the Wright Brothers' plane and fitting it with satnav and radar. If you see what I (somewhat whimsically) mean.
I hasten to add that I'm not trying to be unduly critical. In fact, I'm a huge supporter of spaceflight, manned and unmanned. As a kid I used to eagerly anticipate the day when NASA might announce that they'd be sending people back to the Moon. My 10 year old daydreams would lead me to wonder what we might find, the day we finally made it to Mars. After all, we went to the Moon over ten years before I was born. Why shouldn't we be able to get to Mars within my lifetime. Sadly, that announcement never came. More and more often, people would speak wistfully of the days "when we went to the Moon", as if talking about a childhood holiday to Brighton or Lyme Regis. Where was the passion? Where was the drive to go back? To go further?
Unfortunately, now people are genuinely talking about going back, I find myself confused, wondering why they're using essentially the same methods they used 40 years ago. Don't get me wrong, here. Apollo worked. It worked quite successfully (despite early mishaps). A brand new 4-door family saloon car these days probably contains more sophisticated technology than those intrepid astronauts used to first set foot on the Moon, and I wouldn't dare belittle or diminish their achievement in any way. But... haven't we progressed at all?
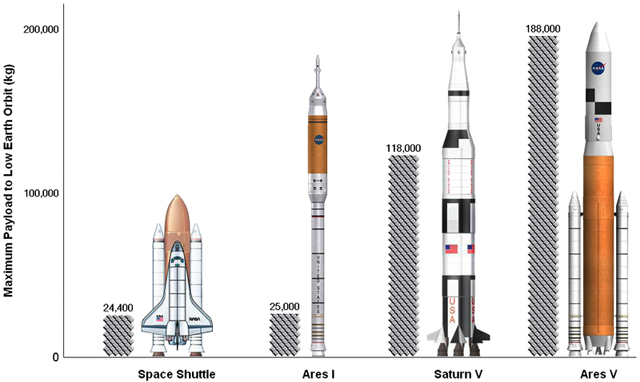
Perhaps the thing I find most puzzling is exactly how much of this thing is going to essentially be disposable! The Saturn V that launched the Apollo missions was then, and still is now, the largest and most powerful launch vehicle ever constructed. The Constellation Program proposes to use not one, but two rockets per mission. One (Ares I) to carry the crew, and one (Ares V) to carry the cargo. Ares V will be by far and away the largest rocket ever. EVER. But really, in this age of recycling and conservation, is this really viable? Weren't we supposed to be working on fully reuseable spacecraft by now?
Spaceplanes were being developed. They were, until their budgets were cut, or their programs were cancelled. The X-33 and it's successor, the Orbital Space Plane should have been practically ready to fly by now. Instead, after investing hundreds of millions of dollars, the plugs were pulled, and these concepts were relegated to remain, simply, as concepts. The cancellation of the Orbital Space Plane was largely a reaction to the Space Shuttle Columbia disaster in 2003. Before the ill-fated STS-107, the Shuttles were deemed viable for use right up to about 2030. Afterwards, it was decided that an Apollo-style capsule would be safer for the crew. The days of the Shuttle were numbered.
Unfortunately, this leaves us having taken one step forward and two steps back. Recreating the Apollo missions seems to be the "safe" option, even inspite of the fact that the Apollo mission itself wasn't without its setbacks -- most notably, the catastrophe that was Apollo 1. Back then, that caused a major rethink and considerable reworking of the technology in order to prevent the same failure happening again. The result was that the remainder of the Apollo program (until it too was cancelled) was a resounding success (with one "sucessful failure"). Sadly, it seems that if the Apollo 1 incident happened tomorrow, it would probably see the cancellation of the entire Apollo program.
T Tauri Stars

The first star recognised to have only just formed, T Tauri lies around 400 light years away on the edge of just such a dense molecular cloud. Only about a million years since it first ignited, T Tauri isn't fully condensed yet. Being the first of its kind recognised (the "prototypical example"), it gives its name to an entire class of young Sun-like stars: the T Tauri stars.
Being young stellar objects (at most, only a few million years old), T Tauri stars aren't yet at hydrostatic equillibrium, so they're much puffier than adult stars. Being so puffy, they're highly convective, giving them potent magnetic fields which can unleash violent x-ray flares. They also don't yet fuse hydrogen in their cores. That won't happen until they're fully condensed. Young stars like T Tauri also have a powerful stellar wind. This stellar wind blows away any excess gas, preventing the star from growing further in size and fixing its initial mass.
T Tauri itself is actually part of a system containing at least 3 stars, though it's hard to tell because of all the dust still surrounding the system. Being so young, these stars haven't had time to settle into a stable orbit yet. Their orbits are still quite erratic. As a result, there's actually a chance that T Tauri itself is in the process of being ejected from the system after a recent brush with one of its siblings. Perhaps a similar thing once happened to the Sun, explaining why our star is a loner in a Universe full of multiple star systems.
On the Curious Clouds of Planet Venus
 Evidently, Venus has once again managed to flummox experts across the planet with its latest stunt -- the appearance of a bright spot amongst the clouds in its southern hemisphere. An awful lot of people are talking about it, but no one really seems to know what's going on over there!
Evidently, Venus has once again managed to flummox experts across the planet with its latest stunt -- the appearance of a bright spot amongst the clouds in its southern hemisphere. An awful lot of people are talking about it, but no one really seems to know what's going on over there!First discovered by an amateur astronomer named Frank Melillo, from Holtsville NY* on July 19th and later confirmed by ESA's Venus Express probe, the cause of the spot is unclear. Which is to say that the experts are clueless. ESA estimate that the spot probably formed about 4 days before it was first spotted from Earth. Since then, being dragged by the venusian winds, it's elongated drastically. It probably won't last long before whatever it is diffuses into the atmosphere, causing it to fade. But the biggest question remains that no one knows why it exists to begin with.
We do know one thing about Venus's clouds. The most reflective patches are droplets of pure sulfuric acid (which start to crystallise at higher altitudes). Assuming there isn't any drastically different atmospheric chemistry going on here, it would be a safe bet that this spot may be a concentration of sulfuric acid. The spot seems to be quite high up in the atmosphere. Around 65-70km high, which is a region where solar ultraviolet can drive a lot of chemistry. If there was a sudden upsurge of sulfur dioxide (SO2) into this part of the atmosphere, it could happily react with the water vapour found up there and make a bright patch of sulfuric acid.
I hasten to add, that this is speculation on my part, and not being a planetary scientist I could easily be wrong. Though if this was indeed what happened, a sudden upwelling could conceivably be caused by a volcano. I've written before about how no direct evidence has been found for present day volcanoes on Venus. Could we be looking at the first sign of volcanism? It would certainly be quite exciting -- settling the matter of how Venus's acidic clouds are replenished, and helping explain a lot of the planet's chemistry!
Unfortunately, there are quite a few things wrong with that idea. Firstly, for an eruption to propel gasses that high into the atmosphere, it would have to be incredibly powerful. Probably comparable to Krakatoa's eruption back in 1883. On the other hand, Venus's atmosphere is much much denser than Earth's, and venusian volcanoes aren't thought to work that way. They're expected to be more akin to basaltic shield volcanoes like Mauna Loa in Hawaii. Basaltic volcanoes produce thin runny lava. They don't cause big explosions. Another problem with the idea is that the spot doesn't lie near any suspected volcanoes, although it's likely to have drifted significantly far from where it originated thanks to those strong venusian winds.
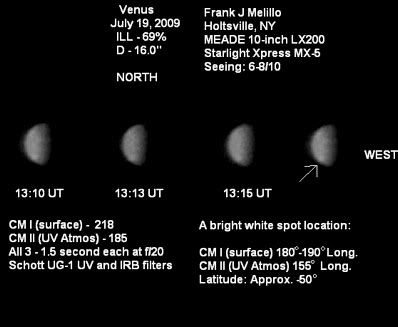
Melillo's original images, showing the first sighting of the bright spot.
Other ideas suggested have included charges particles from the solar wind interacting with Venus's atmosphere, or even random atmospheric currents conentrating material through sheer chance. There's also a possibility there are atmospheric convection currents that we don't yet know about. Perhaps this was some kind of Venusian hurricane, working in a similar way to Jupiter's Great Red Spot.
We've seen bright patches in the atmosphere of Venus before. Periodically, great swathes of those clouds will appear to brighten. Notably in 2007, there were bright patches in both the north and south hemispheres of the planet. Similarly, in early 2008, the entire southern hemisphere of Venus appeared to brighten briefly before fading back to normal.
This spot appears to be different though, with the spot being so focussed, compared to the previous events. It could be something new. We really don't understand Venus at all. Sometimes it even seems as if the more we learn the less we understand.
*I love how, in astronomy, amateurs can make discoveries and observations every bit as important as the professionals do. It's a wonderful science, even if for that reason alone!
Image credits: ESA/MPS & Frank Melillo (respectively)
Beware! Comets ☄
In all fairness, it's widely accepted that there was a giant impact around 65 million years ago, and it's widely (albeit not universally) believed that this event killed off the dinosaurs in the Cretaceous–Tertiary extinction. While it's been hypothesised that other impacts may have been responsible for certain other mass extinctions in Earth's history, Nathan Kaib and Thomas Quinn of Washington University are evidently not so sure.
The two have run some numerical simulations to investigate long period comets. Long period comets are those which come from the very edge of the solar system. Loosely bound by gravity, the Sun is thought to be surrounded by the Oort cloud -- a shell of icy objects. A nearby star passing too close can perturb these cosmic snowballs, causing them to fall towards the Sun and flare into brilliant comets as they start to warm up. If these errant oort cloud objects aren't flung into interstellar space, they can fall into huge elliptical orbits, orbiting the Sun on timescales of thousands to millions of years.

Although it was long believed that the outer reaches of the oort cloud were the main source of such long period comets, Kaib and Quinn's simulations suggest that the actual source is the inner oort cloud. There's a big difference there. A couple of light years of difference, in fact (the outer oort cloud is believed to stretch as far as three light years away from the Sun!). A close encounter with another star can still cause a shower of comets from here -- though even without such a gravitational nudge, it was still found that most long period comets come from the inner and not the outer oort cloud.
The upshot of their simulations, very simply, it's unlikely that more than two or three of these objects could have struck Earth over the past 500 million years, and that's assuming the maximum possible number of oort cloud objects. The (minor) Eocene-Oligocene extinction about 40 million years ago has been suggested before to be the result of a comet shower. If Kaib and Quinn are right, it must have been the most intense cometary shower to have occurred since the Cambrian period! This reduces the chance that extinctions are caused by comet showers. To use Kaib's own words, "...comet showers are probably not likely causes of mass extinction events."
As Jupiter's recent bruise will attest, the giant planets are very helpful in shielding Earth from cometary impacts, deflecting comets or simply bearing the brunt of their impacts so that we don't have to. Interestingly, this means that whatever giant impact occurred to cause the infamous Cretaceous–Tertiary extinction was a purely random event. A fluke. Frankly, I'm not entirely sure how comfortable I am with that...
Image: Comet McNaught, reproduced with kind permission from Chris Picking of Starry Night Skies Photography. His pictures are great. You should go and look at some more!
Reference: Reassessing the Source of Long-Period Comets - Kaib & Quinn (2009)
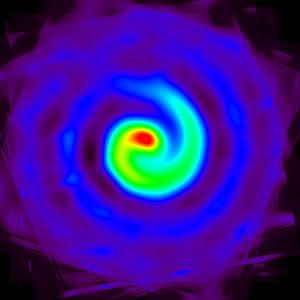
An iris of stars

That pupil however, is a black hole weighing in at 100 million solar masses. Astonishingly though, that bright white iris around it is a flurry of star forming activity. It's thought to be produced by material falling inwards towards the central black hole and is "forming stars at a very high rate," according to NASA's Kartik Sheth.
Interestingly, this is yet more evidence of black holes being helpful towards star formation, even if one or two occasionally get torn apart, devoured or kicked out of the nest...
Source -- Spitzer Space Telescope
Great Oaks, from Tiny Acorns Grow
 Roughly 5 years after NASA's Stardust mission brought home the first ever sample of cometary dust, it's been officially announced. Amongst the chemicals that make up comet Wild 2 is glycine, the simplest amino acid. Looking at the carbon isotopes found in the glycine (it's enriched with carbon-13) confirmed it was formed in space, and likely very old. To quote NASA's Mike Zolensky “We see in this comet that amino acids were forming at the earliest time in our solar system.”
Roughly 5 years after NASA's Stardust mission brought home the first ever sample of cometary dust, it's been officially announced. Amongst the chemicals that make up comet Wild 2 is glycine, the simplest amino acid. Looking at the carbon isotopes found in the glycine (it's enriched with carbon-13) confirmed it was formed in space, and likely very old. To quote NASA's Mike Zolensky “We see in this comet that amino acids were forming at the earliest time in our solar system.”Now amino acids are pretty big news for astrochemical folks like me. To be perfectly honest, the discovery that comets can harbour amino acids didn't come as any surprise to me. It was more of a reassurance that all is right in the Universe. After all, we know amino acids can form in meteorites. We've even found nucleobases in those! Amino acids have been shown to form favourably through good old fashioned thermodynamics, and even chemical theorists not unlike myself have been in on the act of trying to find them in space.
But in comets? The fact that now we know for sure* opens up a few interesting thoughts. One is the old concept of panspermia. And when I say old, I really do mean it. The idea goes back hundreds of years, with various philosophers and scientists pondering whether life could've fallen from the heavens. The most prominent recent proponent of the idea, though, was Sir Fred Hoyle. Hoyle was a firm believer that life itself formed in space, before it arrived to blossom here on Earth. Panspermia tells us that comets could be the seeds from which life grew. Just like any other seed, all they needed was plenty of water and the right amount of sunlight.
Comets really are a hotbed of chemical activity**, containing, in particular, plentiful carbon, oxygen and nitrogen. Reactions between these chemicals could be driven by any number of things. Ultraviolet light, either from the Sun or from other stars can drive chemistry in the outer reaches of the solar system where comets commonly lurk. Alternatively radioactive elements in the comets themselves would create heat as they decayed, causing pockets of warm liquid water for chemistry to take place (coupled with ionising radiation to initiate the chemical reactions therein). No surprise then, that we should find amino acids in them. Considering all of this, it doesn't even seem unreasonable that some kind of primitive life might have formed inside a comet before being delivered to a planet later to fend for itself.
At risk of descending into flagrant speculation (which is all too easily done on such a fascinating topic), the Sun is believed to be surrounded by billions and billions of comets. Some of these comets are occasionally flung straight out of the solar system, to sail off aimlessly into the night. And the Sun is just one of many billions of stars in the Milky Way. Even if only a small fraction of these comets contain the fundamental ingredients of life, it certainly lends a lot of weight to the idea of panspermia. Maybe David Darling was right. Perhaps life really is everywhere.
*And believe me, science is all about knowing things for sure. Don't mind my idle speculations, by the way. That's what a blog's for, after all.
**As I've written about before actually, a lot of molecules were discovered for the first time, anywhere, ever in comets. The brightest optical emission line of the C3 molecule lies somewhere in the violet part of the visible spectrum and, as a result of how it was discovered, is still commonly referred to as the "Comet Head Band!"
On science, beauty and demystification...
"Science takes the romance out of things." This was an offhand comment made to me in conversation at the pub the other night. Is that really what people believe? Apparently, it is. It's regrettable, but I suppose the old stereotype of scientists being unable to appreciate the beauty in things is still going strong. While it's a sad fact that there are a number of scientists I've met who fit this image, it's certainly not true of all of us (indeed, the same is true of many non-scientists too). It may be the case that some scientists might scoff at the concept of beauty and dismissively explain away the natural phenomena which cause aesthetics. In my opinion, these scientists are doing it wrong.
To me, science unveils a myriad worlds of beauty which our fragile minds could scarcely have imagined if we hadn't taken the time to understand how it is that they exist. In turn, understanding how beauty can come about can only serve to heighten the fascination it holds, even for the beauty we're already quite familiar with.
Consider a sunset. There are few people alive who haven't taken at least a fleeting moment to appreciate the majesty of a sunset. The resplendent colours which fill the sky and the long shadows cast across the face of our planet. And if anyone reading this has never stopped to watch a sunset, I heartily recommend that you do. Take a second to appreciate how enthralling nature can be. You won't regret it.
But take a second also, to consider exactly what it is you're seeing. Three million times as massive as the Earth and almost 150 million kilometres away, the Sun is the source of all of these colours. Light from the Sun spans this huge distance at a dazzling speed, reaching planet Earth in just over 8 minutes. As that sunlight reaches our atmosphere it illuminates the billions and billions of molecules which make up the air. Even though air, to us, is completely transparent, the individual photons which make up that light can bounce haphazardly off these molecules, scattering in all directions. The light which is scattered most is blue light, which is what gives our sky the deep cerulean hue we admire so, on a warm summer afternoon. At sunset though, the sunlight reaching us has passed through so much of our atmosphere that there's very little blue left in it. This is why at sunset, the sky is reddest when it's low to the horizon, fading through orange and yellow higher in the sky.
Beauty is not a magic trick. Understanding how it works doesn't detract from its magnificence. If anything, the realisation of the sheer magnitude of what you're seeing should make it all the more captivating. Captivating, and ever so slightly humbling, with the realisation that each of us is just a tiny part of a huge planet, in a huger solar system. In an unimaginably vast Universe.
Just try and tell me that scientific understanding isn't beautiful.

"It does no harm to the romance of the sunset to know a little bit about it."
-- Carl Sagan
To me, science unveils a myriad worlds of beauty which our fragile minds could scarcely have imagined if we hadn't taken the time to understand how it is that they exist. In turn, understanding how beauty can come about can only serve to heighten the fascination it holds, even for the beauty we're already quite familiar with.
Consider a sunset. There are few people alive who haven't taken at least a fleeting moment to appreciate the majesty of a sunset. The resplendent colours which fill the sky and the long shadows cast across the face of our planet. And if anyone reading this has never stopped to watch a sunset, I heartily recommend that you do. Take a second to appreciate how enthralling nature can be. You won't regret it.
But take a second also, to consider exactly what it is you're seeing. Three million times as massive as the Earth and almost 150 million kilometres away, the Sun is the source of all of these colours. Light from the Sun spans this huge distance at a dazzling speed, reaching planet Earth in just over 8 minutes. As that sunlight reaches our atmosphere it illuminates the billions and billions of molecules which make up the air. Even though air, to us, is completely transparent, the individual photons which make up that light can bounce haphazardly off these molecules, scattering in all directions. The light which is scattered most is blue light, which is what gives our sky the deep cerulean hue we admire so, on a warm summer afternoon. At sunset though, the sunlight reaching us has passed through so much of our atmosphere that there's very little blue left in it. This is why at sunset, the sky is reddest when it's low to the horizon, fading through orange and yellow higher in the sky.
Beauty is not a magic trick. Understanding how it works doesn't detract from its magnificence. If anything, the realisation of the sheer magnitude of what you're seeing should make it all the more captivating. Captivating, and ever so slightly humbling, with the realisation that each of us is just a tiny part of a huge planet, in a huger solar system. In an unimaginably vast Universe.
Just try and tell me that scientific understanding isn't beautiful.

"It does no harm to the romance of the sunset to know a little bit about it."
-- Carl Sagan
Horizon
I may have said this before, but the interesting thing about studying astronomy is that you end up holding stars inside your mind. The stars ceased to be mere points of light to me a long time ago. When I look at a night sky now, I see places. Some so close I'm seeing their light from just a few years ago. Some almost unimaginably distant. I'm also aware of the places which I don't see. The ones so far away that their light is far too dim for my feeble human retinas to detect.

The sea itself is a constant reminder of how the Earth moves, playing its role in the vast machinery of the cosmos. Pulled at by the gravity of the Moon, the sea is in constant motion. Buffetted by kinetic friction from the wind. Roiling in turbulent waves, breaking into a billion fractal droplets of water as they crash to the shore, finishing as little but a fine mist of spray to moisten my skin and leave the faint taste of salt on my lips. Countless trillions of water molecules, all in constant motion, spinning and resonating in harmony. Tiny fleeting hexagonal shapes and forms gather within the water, too small to ever be seen, held together by tenuous intermolecular forces, before almost instantly they dissipate and return to the morass of water molecules from whence they came. Nature is a beautiful thing.
So it's now the last day of 2009. It's sometimes nice to take some time out at the end of the year to reflect on things. Gazing to the horizon sometimes has that effect on me. This year has been somewhat tumultuous, but the high points have been the highest of my life. All in all, on reflection, I think 2009 was a fine year. Here's hoping 2010 will be even better!
H three plus
After my last post, I was asked by my friend Alice (from over at the zooniverse*) about what kind of tenuous chemical bonds might form in interstellar space, and why they might be destroyed at higher pressures. I agreed to write a little bit about some astrochemistry, and with me being an ion-molecule person, the subject matter seemed obvious! So with a brief warning that this post might make anyone who's scared of chemistry slightly squeamish, let me elaborate...
 Allow me to introduce the trihydrogen cation, H3+. It's created in certain low pressure environments from plain old molecular dihydrogen. Firstly, you need to knock an electron out of a H2 molecule like so;
Allow me to introduce the trihydrogen cation, H3+. It's created in certain low pressure environments from plain old molecular dihydrogen. Firstly, you need to knock an electron out of a H2 molecule like so;
H2 + [cosmic ray] → H2+ + e- + [cosmic ray]
Cosmic rays** are the best way of doing that. Mainly because they're everywhere and there are a lot of them. That H2+ molecule will then react with the first thing it collides with, and the first thing it collides with is usually another unsuspecting H2 molecule. Then this happens;
H2+ + H2 → H3+ + H
Voila. One H3+ molecule -- one of my favourite little molecules, and the subject of a lot of intense study by a number of extremely intelligent people. It's even been referred to as "A Beautiful Jewel of Nature." H3+ is a fascinating molecule for a number of reasons. For one, it has some rather unusual chemical bonding. In school we're all taught the rule that there are two types of chemical bond. Ionic bonds between positive and negative ions, and covalent bonds where two atoms share two electrons. As with so many rules in chemistry though, this rule can be bent. Electron deficient molecules like H3+ exhibit 3-centre-2-electron bonding. Which is exactly what it sounds like it should be. The three atomic nuclei are bonded together by only two electrons between them all. In this case, being as there are 3 hydrogen nuclei, the electrons distribute themselves evenly across the entire molecule. Weirdly, this means that there aren't three separate bonds in this molecule. The best description really is one single bond spread across three atoms!
As a result, H3+ is rather weakly bound together. Hydrogen is much more comfortable as a H2 molecule and it'll do its best to get rid of one of those atoms and and be that way, if it can. It'll leave a H+ behind on virtually any molecule it encounters, and because a H+ is simply a proton, the reaction is known as "protonation". Incidentally, this is why H3+ is destroyed almost instantly at higher pressures. It only takes one collision to destroy it. In the air that you're breathing, thousands upon thousands of collisions between molecules are happening every second. The effect is that H3+ is a potent "protonating agent" which sets off a chain reaction of interstellar ion-molecule chemistry.*** For instance, supposing H3+ was to collide and react with an oxygen atom;
H3+ + O → OH+ + H2
The oxygen steals a proton, and leaves a hydrogen molecule to go off about its business elsewhere. This gives an OH+ molecule, which will happily react further with more hydrogen;
OH+ + H2 → OH2+ + H
OH2+ + H2 → OH3+ + H
If at any point, one of these molecules were to absorb a stray electron (like the one kicked off that hydrogen molecule right back at the start), it'll break it apart like a fortune cookie. All of that energy needs to go somewhere, and in small gaseous molecules, it's just too much. The molecule can't dissipate it all quickly enough, so the bond falls apart with all of that energy. This has an interesting effect when an electron is absorbed by one of those OH3+ molecules;
OH3+ + e- → H2O + H
And there you have it. H2O. Water. The third most common molecule in the Universe. This H3+ initiated reaction is how water is formed in interstellar space. Pretty cool, huh? Similar H3+ reactions happen to all sorts of other things, from carbon atoms to large polyatomics. But I've talked enough chemistry for one day. Instead, I'll leave you with a page from my notebook. CH3OH, by the way, is methanol, and as any radio astronomer will tell you, methanol too is seen almost everywhere in interstellar space.

*Welcome to the zooniverse, where all your dreams come true... niverse.
**Incidentally, a cosmic ray isn't actually a "ray" at all. They're particles. Mostly they're protons or sometimes α-particles, travelling at ridiculously high velocities. With such speed comes energy, such that a cosmic ray barely even noticed the fact that it's ionised a molecule. A bit like a subatomic hit-and-run, if you will.
***Technically speaking, H3+ is one of the most powerful acids known for just that reason. That's all a classical acid is -- a molecule which donates a proton to another molecule.
 Allow me to introduce the trihydrogen cation, H3+. It's created in certain low pressure environments from plain old molecular dihydrogen. Firstly, you need to knock an electron out of a H2 molecule like so;
Allow me to introduce the trihydrogen cation, H3+. It's created in certain low pressure environments from plain old molecular dihydrogen. Firstly, you need to knock an electron out of a H2 molecule like so;Cosmic rays** are the best way of doing that. Mainly because they're everywhere and there are a lot of them. That H2+ molecule will then react with the first thing it collides with, and the first thing it collides with is usually another unsuspecting H2 molecule. Then this happens;
Voila. One H3+ molecule -- one of my favourite little molecules, and the subject of a lot of intense study by a number of extremely intelligent people. It's even been referred to as "A Beautiful Jewel of Nature." H3+ is a fascinating molecule for a number of reasons. For one, it has some rather unusual chemical bonding. In school we're all taught the rule that there are two types of chemical bond. Ionic bonds between positive and negative ions, and covalent bonds where two atoms share two electrons. As with so many rules in chemistry though, this rule can be bent. Electron deficient molecules like H3+ exhibit 3-centre-2-electron bonding. Which is exactly what it sounds like it should be. The three atomic nuclei are bonded together by only two electrons between them all. In this case, being as there are 3 hydrogen nuclei, the electrons distribute themselves evenly across the entire molecule. Weirdly, this means that there aren't three separate bonds in this molecule. The best description really is one single bond spread across three atoms!
As a result, H3+ is rather weakly bound together. Hydrogen is much more comfortable as a H2 molecule and it'll do its best to get rid of one of those atoms and and be that way, if it can. It'll leave a H+ behind on virtually any molecule it encounters, and because a H+ is simply a proton, the reaction is known as "protonation". Incidentally, this is why H3+ is destroyed almost instantly at higher pressures. It only takes one collision to destroy it. In the air that you're breathing, thousands upon thousands of collisions between molecules are happening every second. The effect is that H3+ is a potent "protonating agent" which sets off a chain reaction of interstellar ion-molecule chemistry.*** For instance, supposing H3+ was to collide and react with an oxygen atom;
The oxygen steals a proton, and leaves a hydrogen molecule to go off about its business elsewhere. This gives an OH+ molecule, which will happily react further with more hydrogen;
OH2+ + H2 → OH3+ + H
If at any point, one of these molecules were to absorb a stray electron (like the one kicked off that hydrogen molecule right back at the start), it'll break it apart like a fortune cookie. All of that energy needs to go somewhere, and in small gaseous molecules, it's just too much. The molecule can't dissipate it all quickly enough, so the bond falls apart with all of that energy. This has an interesting effect when an electron is absorbed by one of those OH3+ molecules;
And there you have it. H2O. Water. The third most common molecule in the Universe. This H3+ initiated reaction is how water is formed in interstellar space. Pretty cool, huh? Similar H3+ reactions happen to all sorts of other things, from carbon atoms to large polyatomics. But I've talked enough chemistry for one day. Instead, I'll leave you with a page from my notebook. CH3OH, by the way, is methanol, and as any radio astronomer will tell you, methanol too is seen almost everywhere in interstellar space.

*Welcome to the zooniverse, where all your dreams come true... niverse.
**Incidentally, a cosmic ray isn't actually a "ray" at all. They're particles. Mostly they're protons or sometimes α-particles, travelling at ridiculously high velocities. With such speed comes energy, such that a cosmic ray barely even noticed the fact that it's ionised a molecule. A bit like a subatomic hit-and-run, if you will.
***Technically speaking, H3+ is one of the most powerful acids known for just that reason. That's all a classical acid is -- a molecule which donates a proton to another molecule.
Something from Nothing
Now, when most people think about space, they imagine it to be a total vacuum. Completely empty of anything whatsoever. The thing is, that's not quite true. Those spaces between the stars aren't quite as empty as you might think. In fact, space is rather clumpy. Clouds of gas and dust stir, turbulent in the darkness. Patches just a few astronomical units in size. Vast by our standards, but on the scale of an entire galaxy, tiny. This is the so-called "small scale structure" of the interstellar medium.
These patches drift between the stars, clumping together with other patches of material or being eroded away by ultraviolet light. The ultraviolet drives active photochemistry. Strange molecules react and evolve, some of them sticking to the surfaces of dust grains. Tenuous chemical bonds are formed, which would be destroyed instantly at the sort of pressures found here on Earth. Not just molecules are found in these regions, but atoms and ions. Precisely what's happening in these regions is still being discovered. They can be studied by looking at emission from neutral hydrogen at radio frequencies, or by looking for absorption lines in starlight. Some, known affectionately as "infrared cirrus," emit infrared light too.
The image below shows a Bok globule (somewhere in the NGC 281 star forming region). A small clump of dense gas and dust, slowly collapsing into stars. It's illuminated from behind by red light; light from hydrogen atoms being ionised by newly born stars. Take a look at all of those patches around it. All of the clumps and fine filaments. Here and there, you can see stars whose light is shining through the globule. Their light appears redder than the others, because of the blue wavelengths of light being absorbed or scattered, unable to penetrate through the cloud. The process is known as extinction, and it's extremely useful in studying these clouds. Looking at the starlight that's passed through these interstellar clouds, you can see all of the lines in the spectrum where the starlight's being absorbed by atoms or molecules. And not all of those absorptions can be easily identified. That's the hard part. And when I say the hard part, what I really mean is the fun part!

Perhaps the one thing that still amazes me the most is -- that globule of gas and dust. You can see it. It's obviously there, dark against the background, blocking out starlight. But that cloud is still essentially vacuum by terrestrial standards. The highest vacuum ever created on planet Earth is still orders of magnitude denser than that cloud which you see in the image above. Chemical reactions in these clouds occur on timescales of years and individual molecules can travel for miles without encountering even as much as a stray hydrogen atom. That Bok globule may be 50 times the mass of the Sun. To us though, it's mostly made of nothing at all. Look again at that image, and take a moment to think about that.
I love astronomy. Sometimes it's nice to ramble a little about the kind of science I actually do myself...
Image: Bok Globule in NGC 281 - NASA, ESA, The Hubble Heritage Team STScI/AURA (P. McCullough) - There's a fuller version of the image here!
These patches drift between the stars, clumping together with other patches of material or being eroded away by ultraviolet light. The ultraviolet drives active photochemistry. Strange molecules react and evolve, some of them sticking to the surfaces of dust grains. Tenuous chemical bonds are formed, which would be destroyed instantly at the sort of pressures found here on Earth. Not just molecules are found in these regions, but atoms and ions. Precisely what's happening in these regions is still being discovered. They can be studied by looking at emission from neutral hydrogen at radio frequencies, or by looking for absorption lines in starlight. Some, known affectionately as "infrared cirrus," emit infrared light too.
The image below shows a Bok globule (somewhere in the NGC 281 star forming region). A small clump of dense gas and dust, slowly collapsing into stars. It's illuminated from behind by red light; light from hydrogen atoms being ionised by newly born stars. Take a look at all of those patches around it. All of the clumps and fine filaments. Here and there, you can see stars whose light is shining through the globule. Their light appears redder than the others, because of the blue wavelengths of light being absorbed or scattered, unable to penetrate through the cloud. The process is known as extinction, and it's extremely useful in studying these clouds. Looking at the starlight that's passed through these interstellar clouds, you can see all of the lines in the spectrum where the starlight's being absorbed by atoms or molecules. And not all of those absorptions can be easily identified. That's the hard part. And when I say the hard part, what I really mean is the fun part!

Perhaps the one thing that still amazes me the most is -- that globule of gas and dust. You can see it. It's obviously there, dark against the background, blocking out starlight. But that cloud is still essentially vacuum by terrestrial standards. The highest vacuum ever created on planet Earth is still orders of magnitude denser than that cloud which you see in the image above. Chemical reactions in these clouds occur on timescales of years and individual molecules can travel for miles without encountering even as much as a stray hydrogen atom. That Bok globule may be 50 times the mass of the Sun. To us though, it's mostly made of nothing at all. Look again at that image, and take a moment to think about that.
I love astronomy. Sometimes it's nice to ramble a little about the kind of science I actually do myself...
Image: Bok Globule in NGC 281 - NASA, ESA, The Hubble Heritage Team STScI/AURA (P. McCullough) - There's a fuller version of the image here!
Try not to breathe
 Well, this is intriguing. One of the defining characteristics of a lot of life on Earth is the need for oxygen. Oxygen molecules are a ready source of energy that macroscopic lifeforms like you and I take full advantage of. Mind you we've found all sorts of microorganisms (mostly archaea) which can survive without any oxygen at all. Some live off things like sulfur, while others like to digest methane. For a long time, it was believed that complex animals can't survive without a supply of O2 to breathe. Until now.
Well, this is intriguing. One of the defining characteristics of a lot of life on Earth is the need for oxygen. Oxygen molecules are a ready source of energy that macroscopic lifeforms like you and I take full advantage of. Mind you we've found all sorts of microorganisms (mostly archaea) which can survive without any oxygen at all. Some live off things like sulfur, while others like to digest methane. For a long time, it was believed that complex animals can't survive without a supply of O2 to breathe. Until now.This squiddy looking creature is called loriciferi. It can grow to about a millimetre in size, and it's a newly discovered species. One of three species discovered at the bottom of the Mediterranean in the sediment of the L'Atalante basin, some 200km West of Crete. The basin is around 3.5km deep and almost completely devoid of oxygen.
These new loriciferans are the first complex animals ever found which can live without oxygen. Unfortunately, none of the survived the journey up to the surface, but their eggs did. Not just that, but those eggs successfully incubated and hatched without any oxygen at all. The most remarkable thing about this is that animals have always used oxygen. Some believe that oxygen is what gave life the supply of energy it needed to grow into large complex forms to begin with. The loriciferans, however, have evolved back to a state where they don't need it anymore. And that's pretty incredible. Maybe the so called "dead zones" in the oceans, where oxygen is scarce, might not be quite so dead after all...
The astrobiological implication here is obvious. Complex life doesn't need oxygen to survive. If for whatever reason, the oceans on worlds like Europa turn out to be anoxic, there's still hope that complex life might exist there. And that's really quite inspiring!

Mysterious fadeouts...
Remember Epsilon Aurigae? Well, it looks like the mystery behind it is at least partially solved! Since 1821, the star has been observed to drop in brightness every 27 years. Now, by using the CHARA array at Georgia State University, the reason for that dimming has actually been imaged for the first time. And it's a little creepy.
Described by the astronomers who worked on it is a "terrifying image, like something from a Tolkein book," the exact nature of this dark object is still essentially unknown. It's almost certainly a disk of some material, seen edge on. This disk is orbiting the central star, Epsilon Aurigae and causing the periodic dimming seen for almost 200 years... but what exactly might lurk inside the disk is still unknown. Theories range from it being a smaller star, swathed in dust, to a stellar mass black hole in the system.
So this, then, is another of those cases where answering one question leads to an awful lot more questions. Fascinating questions.
There's more on the story over at Universe Today!
EDIT--
Also, some deliciously fine details including some light curves, courtesy of Cosmic Variance (seeing as evidently, Sean Carroll was one of the scientists on the team), and a nice artist's impression from NASA JPL!

Described by the astronomers who worked on it is a "terrifying image, like something from a Tolkein book," the exact nature of this dark object is still essentially unknown. It's almost certainly a disk of some material, seen edge on. This disk is orbiting the central star, Epsilon Aurigae and causing the periodic dimming seen for almost 200 years... but what exactly might lurk inside the disk is still unknown. Theories range from it being a smaller star, swathed in dust, to a stellar mass black hole in the system.
So this, then, is another of those cases where answering one question leads to an awful lot more questions. Fascinating questions.
There's more on the story over at Universe Today!
EDIT--
Also, some deliciously fine details including some light curves, courtesy of Cosmic Variance (seeing as evidently, Sean Carroll was one of the scientists on the team), and a nice artist's impression from NASA JPL!

Volcanic Shenanigans
 The volcano Eyjafjallajökull (pronounced EYE-ah-FYAT-la-JO-kootl) erupted on Wednesday, spewing a huge plume of ash 8km into the atmosphere. Winds have since blown this ash into a huge cloud which is currently covering Northern Europe, almost in its entirety. Seemingly, while volcanoes normall tend to produce some amount of ash, it's been exacerbated in this case by Eyjafjallajökull being under a glacier. Hot magma meeting cold ice causes an immense burst of steam as the water is vapourised instantly. Unfortunately, so it would seem, that burst of steam has also served to aerosolise additional volcanic ash, meaning that Eyjafjallajökull is one messy beast. The ash itself, is essentially finely powdered volcanic rock. Silicates, mostly.
The volcano Eyjafjallajökull (pronounced EYE-ah-FYAT-la-JO-kootl) erupted on Wednesday, spewing a huge plume of ash 8km into the atmosphere. Winds have since blown this ash into a huge cloud which is currently covering Northern Europe, almost in its entirety. Seemingly, while volcanoes normall tend to produce some amount of ash, it's been exacerbated in this case by Eyjafjallajökull being under a glacier. Hot magma meeting cold ice causes an immense burst of steam as the water is vapourised instantly. Unfortunately, so it would seem, that burst of steam has also served to aerosolise additional volcanic ash, meaning that Eyjafjallajökull is one messy beast. The ash itself, is essentially finely powdered volcanic rock. Silicates, mostly.The volcanic ash is stopping planes from flying because of the way jet engines work. Because jet engines suck in air, they'd also suck in ash, with the end result being a fine coating of volcanic glass on the inside of the jet turbines. Needless to say, this isn't good. Volcanic ash has been seen to cause aircraft engines to fail before, and while it's inconvenient to be stuck on the ground, it's far preferable to falling out of the sky!
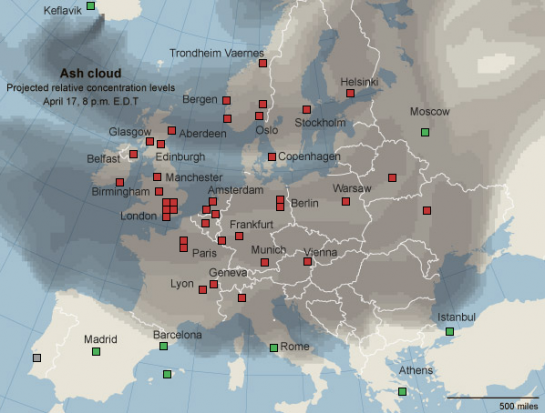
Airports closed due to the ash cloud are shown in red.
So the end result is a huge closure of airports across Europe. Bloody typical. What happens when I'm due to go on my first observing run? "One of the largest disruptions in commercial aviation history" happens. That's what.
Perhaps the most interesting thing going on though, is that the skies have been eerily empty for days now. No jet trails at all. In fact, nothing. No clouds either, and a light haze near the horizon. I'd initially thought there might be lots of clouds, because of the sulfur compounds released by the volcano. Sulfurous aerosols (particularly sulfate and methyl sulfonate) have been shown to nucleate cloud particles, seeding clouds and causing rainfall. But there are no clouds at all. Perhaps all the water has already fallen from the air by the time it reaches this far inland.
EDIT-- Actually, there are indeed clouds now, so I may be wrong on some of this.
Despite all the furore that's normally associated with the matter, you have to wonder precisely what the environmental effect of all of this might be. European air traffic, it has to be said, contributes a large amount of carbon dioxide to the atmosphere. Much more on a daily basis than a volcanic eruption. What's more, sulfur compounds in the atmosphere will likely serve to increase Earth's albedo (albeit only fractionally). The albedo of a planet means how much light its atmosphere reflects. All of the sulfur in Venus's atmosphere gives it one of the highest albedos in the Solar system and is the reason why it reflects so much sunlight and shines so brightly in the evening sky. There's even the possibility that this volcano might help to cool the Earth's northern hemisphere slightly*. Volcanic eruptions in the past have managed to cool the average temperature at the Earth's surface by up to half a degree in the past century -- which may not sound like a lot, but on a global scale, that's rather significant!
Anyway, I'd better call the airline and find out what my travel plans are. I suspect the next few days are going to be no less worrisome than the last few.
*Specifically, sulfur compounds tend to sit in the stratosphere. This cools the lower atmosphere by reflecting away sunlight, but those sulfur compounds also absorb heat from Earth's surface causing the stratosphere to warm slightly. While this may be beneficial for glaciers and ice caps, the effect on the weather would remain to be seen...
Image credits:
"Cracks of Doom" - Skarpi's photostream
The Ash Cloud and Airport Shutdowns - Flowing Data
Radcliffe
I'm currently waiting for a half hour exposure to finish, which will hopefully give me a nice interesting looking stellar spectrum. Ahhh, fresh data.

(I'll write more when I'm less preoccupied and better rested, don't worry...)
EDIT--- I figure I should probably also point out that the photograph is of SALT, not Radcliffe. SALT looks prettier. It also has a primary mirror over 5 times as big!
Earth Day

Happy Earth Day!
If you're not sure what Earth Day is supposed to be all about,
try this. Step outside. Take a deep breath. Taste the air. Whatever
the weather may be, feel the sun on your face, the wind in your
hair or the rain on your skin. Marvel at this planet and all
the myriad ways in which it keeps us alive, and cradles the only
life we know of in this entire Universe.
This tiny crucible.
Our pale blue dot.

Views from the Top

The road up to the telescope domes.

Radcliffe Telescope dome, flanked by the 20 inch and 40 inch telscope domes in the background.

Radcliffe entranceway, with SALT in the background.
Naos
O-type stars are rare. In fact they're very rare. Less than one in every hundred thousand stars formed will be an O-star. The reason they're so rare is that they're incredibly massive. Stars with such immense mass will burn through all of their fuel rapidly, so fast that they can only live for a few million years. An O-star which formed when the dinosaurs became extinct would be long dead by now.
Naos here, is an O-star. With an estimated age of about 4 million years, it's quite old for its type. In fact, it's one of the few O-stars in the galaxy which are visible to the naked eye. Being an extreme blue supergiant with 59 solar masses and a surface temperature of over 42000 kelvins, Naos is actually one of the brightest stars in the Milky Way. It's almost 800 000 times as bright as the Sun, although most of that light is ultraviolet. So much so that in the visible, it's only around 21000 times as bright visually.
Nonetheless, an O-star is a powerful object. If Naos was as close to us as Sirius, it would have an apparent magnitude of -9. That's bright enough to cast shadows on Earth's surface, and easily bright enough to read a book by at midnight! The full moon, for comparison, has a magnitude around -12. Even where Naos is, at around 1090 light years away, when it starts to die in somewhere between a few thousand to a million years, it'll become one of the brightest stars in the sky for a time. Eventually it will explode as a supernova much brighter than the full moon.

It's interesting what you discover sometimes when you're looking for standard stars to calibrate your data with...
Naos here, is an O-star. With an estimated age of about 4 million years, it's quite old for its type. In fact, it's one of the few O-stars in the galaxy which are visible to the naked eye. Being an extreme blue supergiant with 59 solar masses and a surface temperature of over 42000 kelvins, Naos is actually one of the brightest stars in the Milky Way. It's almost 800 000 times as bright as the Sun, although most of that light is ultraviolet. So much so that in the visible, it's only around 21000 times as bright visually.
Nonetheless, an O-star is a powerful object. If Naos was as close to us as Sirius, it would have an apparent magnitude of -9. That's bright enough to cast shadows on Earth's surface, and easily bright enough to read a book by at midnight! The full moon, for comparison, has a magnitude around -12. Even where Naos is, at around 1090 light years away, when it starts to die in somewhere between a few thousand to a million years, it'll become one of the brightest stars in the sky for a time. Eventually it will explode as a supernova much brighter than the full moon.

It's interesting what you discover sometimes when you're looking for standard stars to calibrate your data with...
Radcliffe
 Ladies and gentlemen, this is the Radcliffe Telescope, and it's what I've been eagerly awaiting the use of for quite some time now. Unfortunately, while I'd love to be recording some spectra of the various stars on our target list right now, the weather is refusing to cooperate. With the mountaintop covered in fog, observing is currently off the menu. Sadly. So while I'm waiting for things to clear up, I might as well write a little about the 'scope...
Ladies and gentlemen, this is the Radcliffe Telescope, and it's what I've been eagerly awaiting the use of for quite some time now. Unfortunately, while I'd love to be recording some spectra of the various stars on our target list right now, the weather is refusing to cooperate. With the mountaintop covered in fog, observing is currently off the menu. Sadly. So while I'm waiting for things to clear up, I might as well write a little about the 'scope...Radcliffe is a lovely blend of early 20th century engineering and late 20th century electronics. Five metric tonnes worth of wrought iron telescope, equatorially mounted with another five metric tonnes worth of counterweight. With a 1.9 metre mirror, Radcliffe is essentially an oversized Newtonian telescope, not unlike the one I've had since I was a little kid. It's simply been scaled up. While it works as a cassegrain now, it even still has a Newtonian mounting on it. As you may be starting to guess, Radcliffe is an old telescope.
It wasn't even originally at this site. Named after an English physician, John Radcliffe, it was originally the telescope of the Radcliffe Observatory in Pretoria where it was operated between 1948 and 1972. In 1972, it was moved to its current site at the South African Astronomical Observatory here in Sutherland. Until the mirrors were installed in SALT in 2004, Radcliffe remained the largest telescope in Africa. Indeed, for quite a few years after its construction it was actually the largest telescope in the southern hemisphere, and joint fourth largest telescope in the world. Those days, however, are past and 2 metre class telescopes like Radcliffe are now considered "small telescopes" by professional astronomers. Compared to the shiny new 10 metre class telescopes now dotted around the world, Radcliffe is quite a downbeat piece of equipment with its fully manual operation and its old fashioned mechanisms. And frankly, therein lies its charm.
The most beautiful thing about using an old school telescope like this one is how hands on everything is. A series of buttons on a metal panel move the telescope, the mirror cover has to be opened using a metal crank with a wooden handle, and the sensors need to be cooled by manually refilling the liquid nitrogen tank periodically. It even has a finderscope attached to it, though this hasn't been used for some time. The finder alone is still significantly bigger than many amateur telescopes, mind you. The reason this gorgeous piece of engineering is so hands on is, simply, because it's old. Newer telescopes don't allow you quite so much freedom. I've been told that some telescopes don't really allow you to do anything yourself, being more a matter of clicking the odd button on occasion, or simply telling a telescope operator what to do. Purely for the thrill of being able to manually operate the telescope, it's been a lot of fun working with it so far, as I have no doubt it will continue to be. Provided the weather stops thwarting us!

Take pride in thy geekery!
So apparently, I am a geek. Though sometimes I'm not sure why. Maybe it's because I don't draw the same kind of strict boundaries between work and !work that other people draw... But that's a rant for another time. In any case, for your nerdish enjoyment, I present the Geek Manifesto!
The Geek Manifesto
Rights:
- The right to be even geekier.
- The right to not leave your house.
- The right to not like football or any other sport.
- The right to associate with other nerds.
- The right to have few friends (or none at all).
- The right to have as many geeky friends as you want.
- The right to be out of style.
- The right to be overweight and near-sighted.
- The right to show off your geekiness.
- The right to make an attempt at being as geeky as Matt Young, and the right to fail. (Topher Stumph came quite close, but he too, failed).
- The right to develop serious crushes on Randall Munroe, Shane Carruth & Bo Burnam, as opposed to say… James Franco. (See 10).
- The right to carry a Thesaurus with you at all times, as opposed to an iPhone. (See 10)
- The right to execute shameless self advertisement via the Wikipedia Geek Pride Day page. (See 10).
- The right to falsely assume the surnames Finkleton, Waldman, Stratzer and Krukemeyer.
- The right to quote Firefly, xkcd, or both, whenever at all possible.
- The right to take over the world.
Responsibilities:
- Be a geek, no matter what.
- Try to be nerdier than anyone else.
- If there is a discussion about something geeky, you must give your opinion.
- To save and protect all geeky material.
- Do everything you can to show off geeky stuff as a "museum of geekiness."
- Don't be a generalized geek. You must specialize in something.
- Attend every nerdy movie on opening night and buy every geeky book before anyone else.
- Wait in line on every opening night. If you can go in costume or at least with a related T-shirt, all the better.
- Don’t waste your time on anything not related to geekdom.
- Befriend any person or persons bearing any physical similarities to comic book or sci-fi figures.
- Try to take over the world!
(Source)
In fairness, one should be careful not to confuse a geek with a nerd. There is a subtle difference between the two, as is exemplified by this convenient venn diagram:
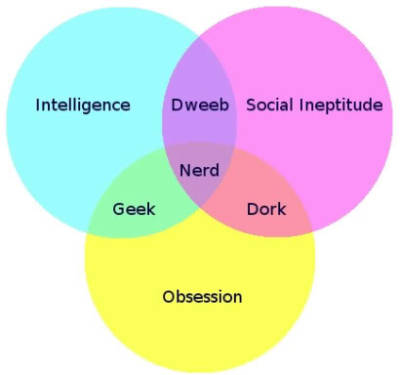
...I just proved my point didn't I? Crap. Oh well, at least I can be proud of it today!
Subscribe to:
Posts (Atom)